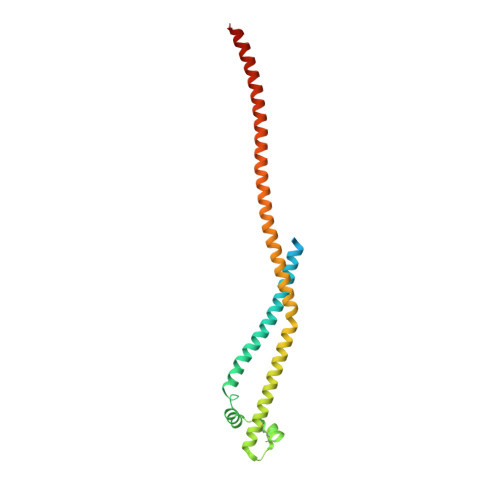Structure of a Three-Dimensional Domain-Swapped Dimer of the Helicobacter Pylori Type Iv Secretion System Pilus Protein Cagl.
Barden, S., Schomburg, B., Conradi, J., Backert, S., Sewald, N., Niemann, H.H.(2014) Acta Crystallogr D Biol Crystallogr 70: 1391
- PubMed: 24816107
- DOI: https://doi.org/10.1107/S1399004714003150
- Primary Citation of Related Structures:
4CII - PubMed Abstract:
A new crystal form of the Helicobacter pylori type IV secretion system (T4SS) pilus protein CagL is described here. In contrast to two previously reported monomeric structures, CagL forms a three-dimensional domain-swapped dimer. CagL dimers can arise during refolding from inclusion bodies or can form spontaneously from purified monomeric CagL in the crystallization conditions. Monomeric CagL forms a three-helix bundle, with which the N-terminal helix is only loosely associated. In the new crystal form, the N-terminal helix is missing. The domain swap is owing to exchange of the C-terminal helix between the two protomers of a dimer. A loop-to-helix transition results in a long helix of 108 amino acids comprising the penultimate and the last helix of the monomer. The RGD motif of dimeric CagL adopts an α-helical conformation. In contrast to the previously reported structures, the conserved and functionally important C-terminal hexapeptide is resolved. It extends beyond the three-helix bundle as an exposed helical appendage. This new crystal form contributes to the molecular understanding of CagL by highlighting rigid and flexible regions in the protein and by providing the first view of the C-terminus. Based on the structural features, a previously unrecognized homology between CagL and CagI is discussed.
Organizational Affiliation:
Structural Biochemistry, Department of Chemistry, Bielefeld University, Universitätsstrasse 25, 33615 Bielefeld, Germany.